
CHAS1 (Chassahowitzka NWR, MI, Lat. 28.7484, Long. -82.5549, Alt. 4.3) Print-Friendly Version Print-Friendly Version (B&W)
Based on the regional haze rule version 2, only 3 years of complete aerosol data (2000-2002) are available in Chassahowitzka NWR during the baseline period of 2000 - 2004. The PM2.5 mass and elemental concentrations are missing from March 1 of 2003 to the end of 2004 due to possible problems with the channel A sampler. Therefore, no CM, soil, and sea salt concentrations and extinction coefficients are available during this time period. In order to take fully usage of the data available, the methodology discussed below is used to to estimate the missing concentrations, and the numbers shown on this page are based on 5 years of aerosol data during the baseline period of 2000 - 2004 after all possible substitutions.
PM2.5 sea salt concentration is estimated as the concentration of chloride ion (Cl-) measured by ion chromatography multiplied by 1.8. If the chloride measurement is below the detection limit, missing or invalid, and the concentration of chlorine (Cl) measured by XRF is above the detection limit, then the PM2.5 sea salt concentration is estimated as the concentration of chlorine (Cl) measured by XRF multiplied by 1.8. If both chloride and chlorine are missing or invalid, PM2.5 sea salt concentration is not available. Otherwise, PM2.5 sea salt concentration is set to be zero.
A fairly good correlation has been found between PM2.5 and PM10 based on the measurements in 2000-2002 as shown in the figure below. For 2003 and 2004 when PM2.5 mass is missing, PM2.5 is estimated as PM10/1.5, and coarse mass (CM) concentration is then calculated as PM10 - PM2.5.

Figure 1 Relationship between measured PM10 and PM2.5 in 2000-2002
Fine soil concentration = PM2.5 – [Ammonium Sulfate] – [Ammonium Nitrate] – [OMC] – [EC] – [Sea Salt]. If the calculated fine soil is less than zero, it is set to be zero.
As shown in Figure 2, the overall average total light extinction coefficient (Bext) is 82.7 Mm-1 (Visual Range ~ 55 Km; Deciview ~ 20). The average PM2.5 mass concentration is 9.2 mg/m3. The average contributions of the major aerosol components to Seney haze are particulate sulfate 54.9%, nitrate 6.0%, organic matter (OMC) 14.3%, elemental carbon (light absorbing carbon, LAC) 5.7%, fine soil 1.2%, sea salt 0.8%, and coarse mass (CM) 3.8%.

Figure 2 Average contributions of major aerosol chemical components to light extinction (Based on data available in 2000-2004)
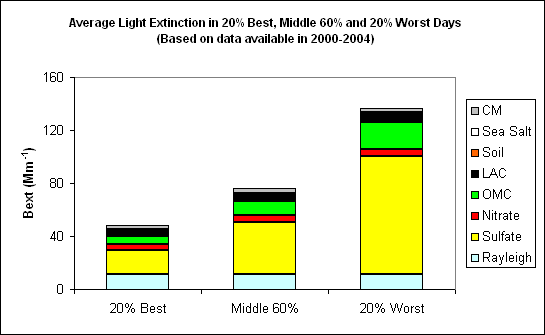
Figure 3 Average contributions of major aerosol chemical components to light extinction in 20% best, middle 60% and 20% worst days (Based on data available in 2000-2004)
As Figure 3 indicates, the average light extinction coefficient during the 20% worst days is 136.8 Mm-1, which is about 2.9 times of the value of 48.0 Mm-1 during the 20% best days and 1.8 times of the value of 75.9 Mm-1 during the middle 60% days. Sulfate is the largest aerosol contributor to light extinction during the 20% worst days, with a contribution of ~ 65%. OMC also contributes about 15% to light extinction during the 20% worst visibility days.
Figure 4 suggests that the highest occurrence of the 20% worst days happened in February, in which ~ 28% of the sampling days are the 20% haziest days at Chassahowitzka. As shown in Figure 5, in the 20% worst visibility days, sulfate is the largest aerosol contributor to haze with a contribution from ~50% in the winter to over 70% in the summer. OMC also contributes significantly to light extinction during the 20% worst days especially during the winter time (~25-30% in the winter).
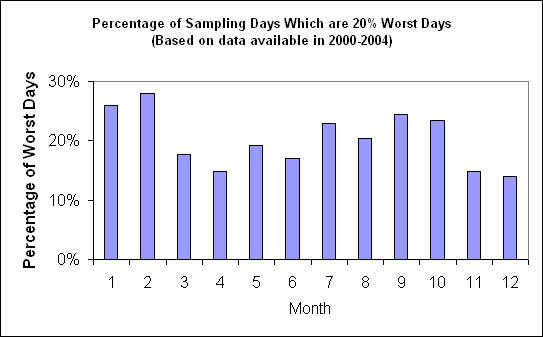
Figure 4 Percentage of sampling days that are 20% worst days in each month (Based on data available in 2000-2004)

Figure 5 Average contributions of major aerosol chemical components to light extinction during 20% worst days in each month (Based on data available in 2000-2004)